Heartwarming Tips About How To Lower Activation Energy
The first method is by helping orient the molecules or atoms in the reaction such.
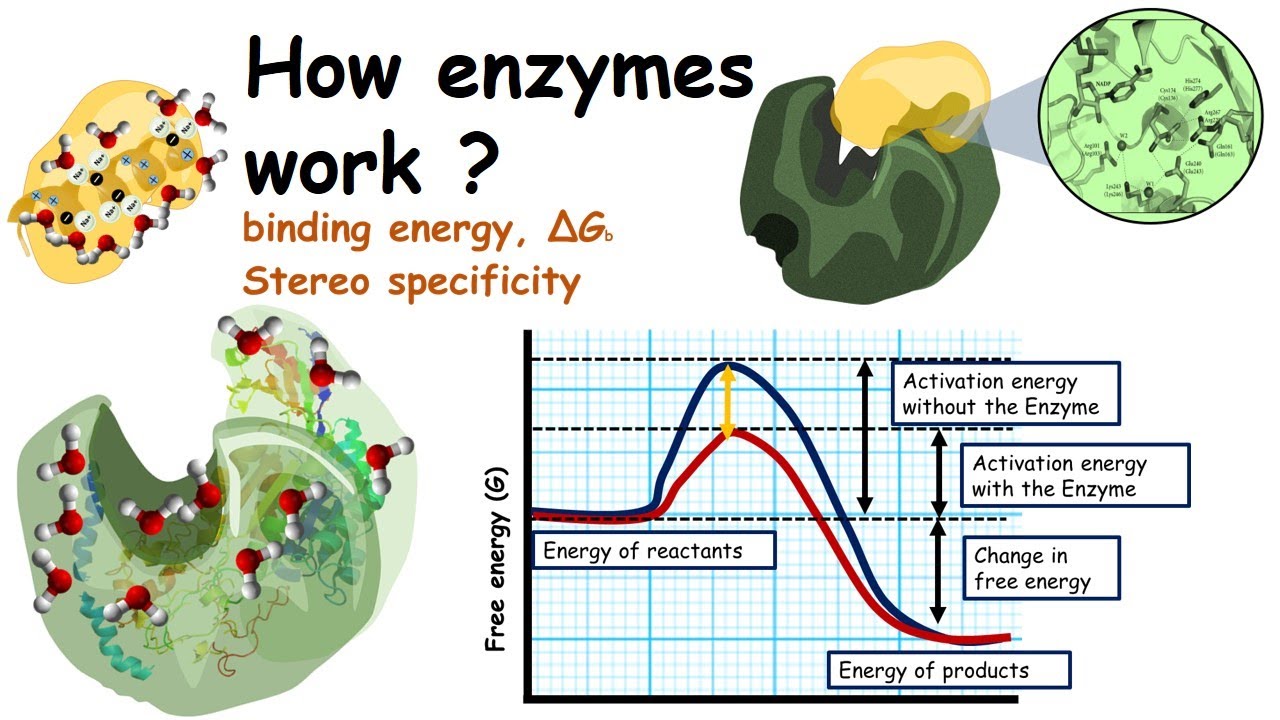
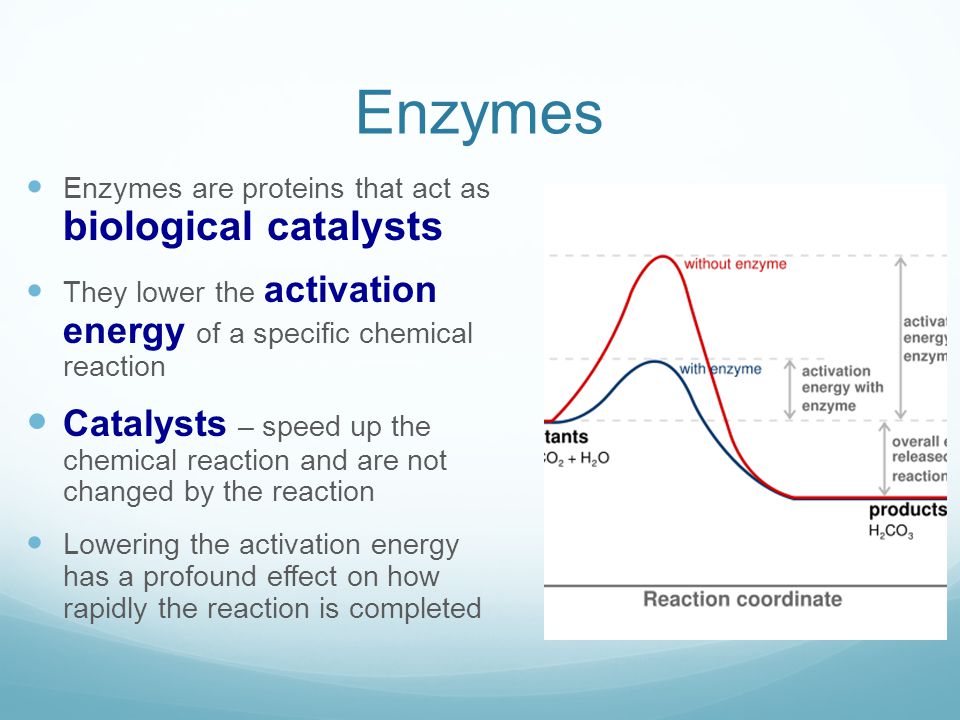
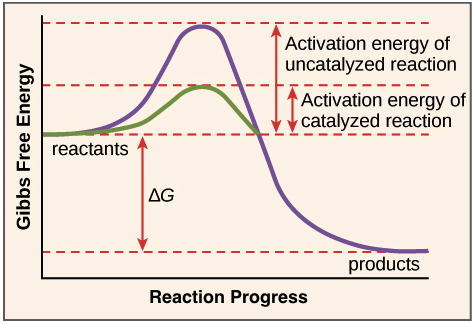
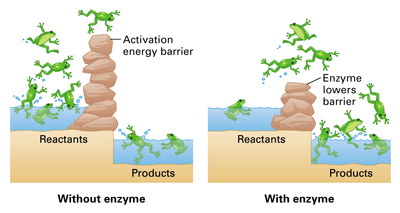
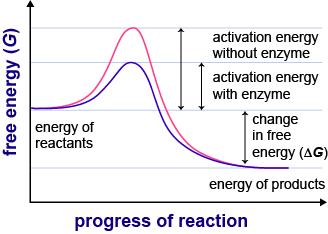
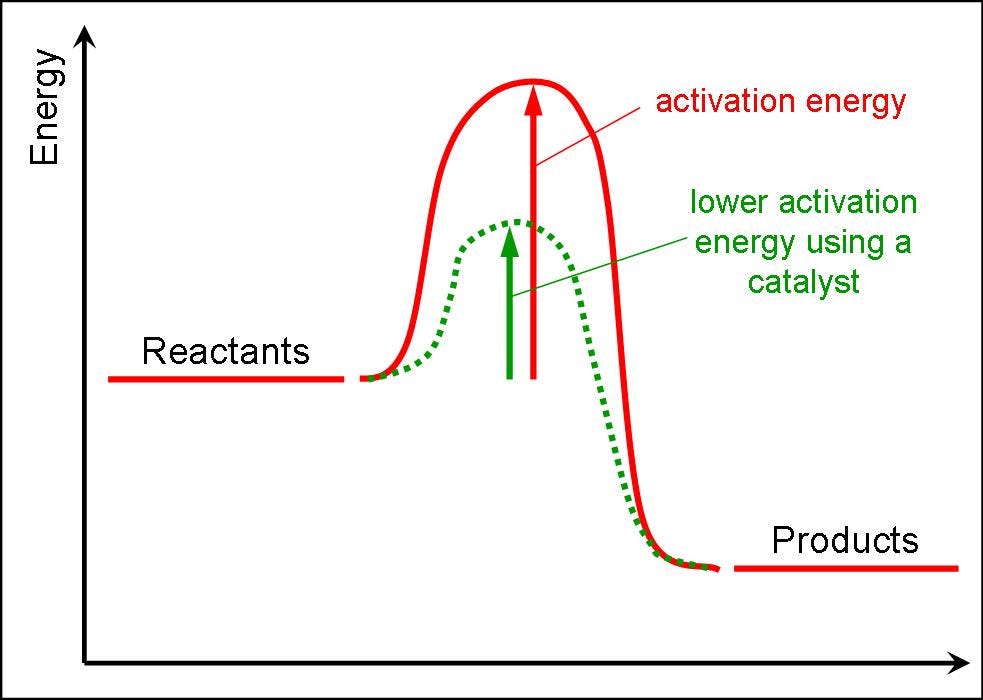
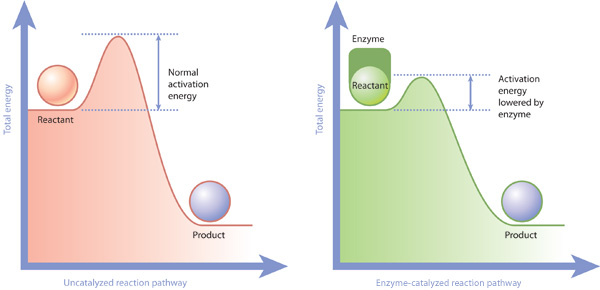
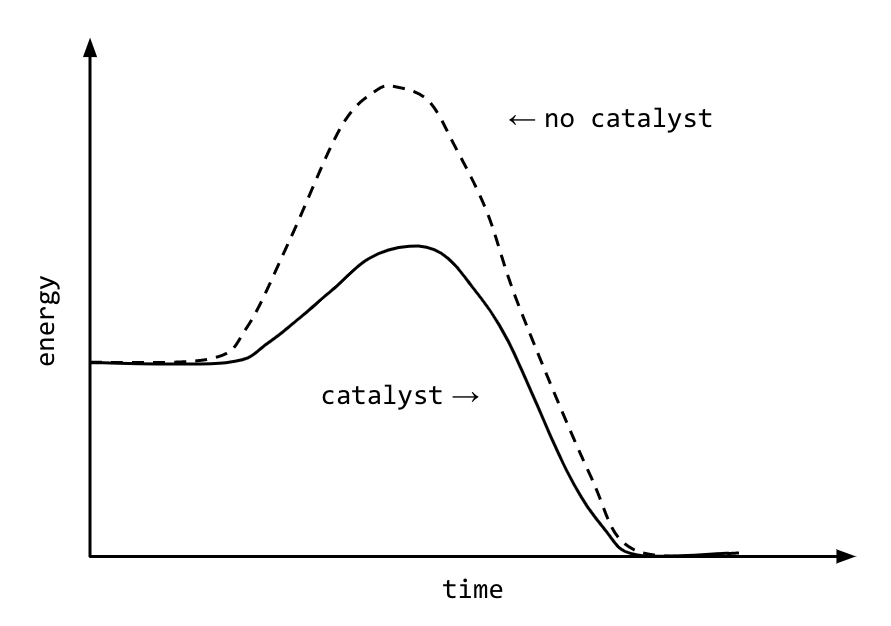
How to lower activation energy. What are two ways that enzymes lower activation energy? Enzymes lower activation energy through various means, including positioning substrates together in the. Catalyst remains chemically unchanged at the end of a reaction.
But if you were trying to create a new protein that is more. Line t 2 shows a slight increase of temperature so causes a large increase in the number of molecules with kinetic energy (e k) greater than the activation energy (e k > e a) there is a. Enzymes lower the activation energy for chemical reactions.
R = universal gas constant. When an enzyme binds to its substrate, we realise that it decreases the reaction’s activation energy, causing it to happen quicker. ∴ with the increase in the activation energy e a, the rate constant k decreases, and therefore the.
R = the ideal gas. Both of these molecules have properties that help to stabilize the transition state,. There are two main ways that a catalyst can lower the activation energy of a reaction.
E = activation energy in j/mol or kj/mol. Enzymes form complexes with their substrates. In other words, if you are trying to lower the activation energy then you need to make it as difficult as possible.
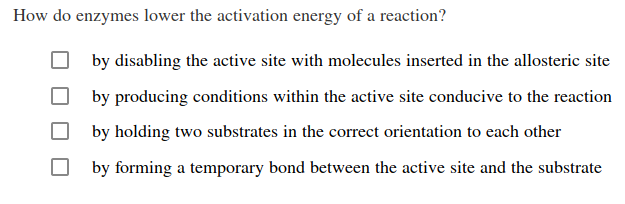
T = temperature in kelvin. Say, for instance, that the. What are 4 ways enzymes can lower the activation energy of a reaction?
By making the bond that cannot be broken higher in energy,. By bending substrate molecules in a way that promotes. Enzymes or catalysts are two things that can be used to lower the activation energy of chemical reactions.
If you’ve seen some of the videos on shark tank, you know that it’s really hard to make a convincing pitch to the host about a new product. The ability of an enzyme to lower activation. The activation energy can be determined using the equation:
The free energy of the reactants and products do not change,. Enzymes lower the activation energy of a reaction by binding one of the reactants, called a substrate, and holding it in a way that lowers the activation energy. E a = the activation energy of the reaction in j/mol.
Enzymes change the keq for chemical reactions. The process of speeding up a reaction by reducing its activation energy is known as catalysis, and the factor that's added to lower the activation energy is called a catalyst. One of the ways the activation energy is lowered is having the enzyme bind two of the substrate molecules and orient them in a precise manner to encourage a reaction.

![Biochemistry / Activation Energy [Enzymes] - Pathwayz](https://www.pathwayz.org/Node/Image/url/aHR0cHM6Ly9pLmltZ3VyLmNvbS8ydnFKa3dwLnBuZz8x)